Dortmund, 14th February 2025
Special connections do not only exist in the romantic sense, they can also occur in research. The editorial team asked ISAS researchers which liaisons in the lab makes their hearts beat faster. For the ISAS Bioimaging research group, the antibody-epitope bond is such a ‘perfect match’. Antibodies are Y-shaped proteins that the immune system produces. They bind to pathogens or foreign molecules to label them or to neutralise them directly. This binding occurs according to a very specific system. Each antibody has a specific region with a highly precise, three-dimensional structure that exactly matches the epitope of the antigen. The epitope is the specific part of the antigen to which the antibody binds. This binding is like the lock-and-key principle. Because of its high specificity, the antibody binds almost exclusively to a specific epitope – and therefore to a specific antigen – making it a 'perfect duo’.
Even minimal changes in the structure of the epitope can influence or even prevent binding. This high level of precision is crucial to the functioning of our immune system. Antibodies must distinguish precisely between the body's own and foreign structures to trigger an effective immune response without attacking healthy tissue.
Immunofluorescence – exciting everyday life at the bioimaging
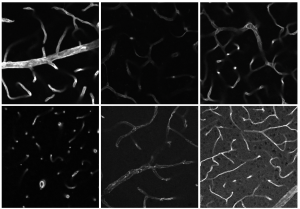
The Bioimaging research group uses this very specific mechanism in its research on an almost daily basis. This is important, for example, for immunofluorescence, a method that can visualise specific proteins in tissues or cells. “Immunofluorescence is one of our standard techniques to visualise and localise defined proteins, lipids, and other target structures in our biological samples,” says Prof Dr Anika Grüneboom, head of the Bioimaging group.
The biologists use antibodies that are coupled to certain fluorescent dyes. When excited, for example by a laser, the fluorophores (see info box) emit light of a specific wavelength. The researchers can then measure these emitted photons (quasi light particles). When the labelled antibodies bind to their target protein, the scientists can use fluorescence microscopes to visualise structures at the subcellular level. This allows them to precisely analyse the spatial distribution of proteins in biological samples.

Prof Dr Anika Grüneboom heads the Bioimaging research group and the research programme 3D Molecular Pathology at ISAS.
© ISAS / Hannes Woidich
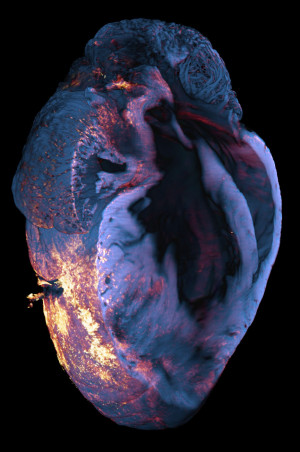
The photo shows the heart of a mouse after a heart attack. It was taken with a light sheet fluorescence microscope. To identify the immune cells of the macrophage type that have migrated into the heart muscle tissue after a myocardial infarction (heart attack), the researchers use the antibody for the CD68 antigen. This antibody is coupled to a bright red fluorescent dye. As soon as the antibody binds to the CD68 antigen of the macrophages, these immune cells can be localised under the light-sheet fluorescence microscope by means of the emitted photons (quasi light particles), as shown here in red orange.
© ISAS / Anika Grüneboom
Fluorophores
A fluorophore is a fluorescent chemical compound that can absorb photons in its initial state. Fluorescence describes the ability of substances to absorb short wavelength light and emit it at a different wavelength. The electrons in a molecule are briefly excited before returning to their original energy level. This releases energy that we perceive as light. To recognise certain structures with a fluorescence microscope, scientists add fluorescent markers or fluorochromes to their samples. These stick to certain structures like a little flag and make them visible under the fluorescence microscope.
3D bioprinting meets NMR
For Dr Themistoklis Venianakis, the answer to the question about special connections is clear. “The combination of 3D bioprinting technology with the method of nuclear magnetic resonance spectroscopy, or NMR for short, is a perfect match,” says the chemist. The NMR Metabolomics research group creates three-dimensional cell cultures using 3D printing. These cultures have to fit into very small tubes so that they can be analysed under physiological, meaning realistic, conditions using NMR. The aim is to investigate metabolic processes quantitatively and qualitatively. The metabolome (the entire set of metabolic products in an organism) can provide information about possible disease in the body. To establish the combination of 3D bioprinting and NMR analysis as an unbeatable combination, Venianakis is currently working on printing structures that are a perfect fit for NMR – and at the same time provide a safe environment for the cells.

Dr Themistoklis Venianakis is a chemist and works as a research associate in the NMR Metabolomics group.
© ISAS

Dr Themistoklis Venianakis uses small tubes for the analysis with the NMR. The milky looking sample consists of alginate and gelatine. It provides a scaffold for the cells. For the cells to survive inside the tube, the nutrient solution must have a neutral pH value. This is indicated by the reddish colour. In an acidic environment, the solution would turn yellow.
© ISAS
Another »perfect match«: alginate and gelatine
According to Venianakis, the biggest challenge is that the material has to be stiff enough for solid structures, but at the same time still mouldable for 3D printing. The perfect match also plays an important role here: alginate, a naturally occurring polysaccharide, provides a solid, unbreakable structure, while gelatine makes the mixture more flexible and softer. The two substances act as a kind of scaffolding for the cells. The 3D printer uses the three components alginate, gelatine and cells to create the structures for the analysis via NMR.
(Anna Becker / Nadine Kuhnen)