Dortmund, 14th February 2020
Iodide salts as the key to sustainable success of biocatalysts in fuel cells? The mass use of fuel cells could make large power plants and high-voltage lines superfluous, make cars run emission-free and turn homeowners into their own electricity producers. But: One type of catalysts - precious metals like platinum - are very expensive and the resources are decreasing more and more. The second type of catalyst would be highly abundant: biological and bio-inspired catalysts. Nevertheless, these natural catalysts have so far hardly been used for energy conversion processes. This is because these catalysts are often so sensitive to oxygen that they stop functioning within a few seconds.
How to solve this? A research team led by Prof. Dr. Nicolas Plumeré from the Ruhr University Bochum (RUB), Dr. Erik Freier and Dr. Ute Münchberg from the Leibniz-Institut für Analytische Wissenschaften in Dortmund (ISAS) and Prof. Dr. Wolfgang Lubitz from the Max Planck Institute for Chemical Energy Conversion in Mülheim dealt with this issue. They report in the renowned journal Nature Communications on 14 February 2020: "Iodide salts make biocatalysts for fuel cells stable".

Infinite protection - so far only in theory
The research group recently discovered that redox-active films can protect bio-inspired and even biocatalysts such as hydrogenases from oxygen. Theoretical models predict that protection against oxygen should last indefinitely. In experiments, however, this protection is only effective for a few hours. "This contradicts our theoretical calculations and cannot be explained, especially considering the lifetime of the same catalyst in an oxygen-free environment," says Plumeré. The latter is up to six weeks with constant turnover.
Combination of methods gets to the bottom of the problem
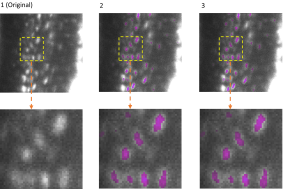
The researchers concluded that either the mechanism for protection against oxygen is not yet understood, or that in addition to deactivation by oxygen, additional harmful processes take place. To investigate this, they combined various methods that allowed them to examine exactly what happens in the protective layer. The combination of confocal fluorescence microscopy and coherent anti-Stokes Raman scattering performed in the laboratory of Erik Freier at ISAS with electrochemistry for the analysis of the protective matrix showed: The protective process leads to an accumulation of hydrogen peroxide damaging the catalytic film.
Suppress hydrogen peroxide formation
The work shows that the splitting of hydrogen peroxide using iodide salts increases the half-life of a hydrogenase for hydrogen oxidation to up to one week at constant turnover, even when exposed to constant high oxygen concentrations. "Overall, our data confirm the theory that redox films make oxygen-sensitive catalysts completely immune to direct deactivation by oxygen," summarizes Plumeré. "However, it is very important to also suppress hydrogen peroxide production in order to achieve complete protection against oxidative stress."
"Our work shows that the simple strategy of adding iodide salts to the electrolyte can be sufficient to significantly reduce the inactivation rates of biocatalysts," said the researchers. In their opinion, this will enable the widespread implementation of other electrocatalytic processes in real applications. This also includes energy conversion processes such as solar fuel generation through carbon dioxide reduction and the electrosynthesis of fine or basic chemicals such as ammonia.
Funding
The work was funded by the German Research Foundation as part of the Ruhr Explores Solvation Resolv Cluster of Excellence (EXC-2033 - project number 390677874) and the Shields project (PL 746/2-1) and by the European Research Council as part of Starting Grant 715900. Further support came from the Max Planck Society, the China Scholarship Council and the Ministry of Culture and Science of North Rhine-Westphalia, the Governing Mayor of Berlin - including Science and Research, and the Federal Ministry of Education and Research and the Leibniz Research Cluster (031A360E).
Original publication
Huaiguang Li, Ute Münchberg, Alaa A. Oughli, Darren Buesen, Wolfgang Lubitz, Erik Freier, Nicolas Plumeré: Suppressing hydrogen peroxide generation to achieve oxygen-insensitivity of a [NiFe] hydrogenase in redox active films, in: Nature Communications 2020, DOI: 10.1038/s41467-020-14673-7
Further Links
Read here the article on the topic, which was published in Nature Communications on 14.02.2020.
Click here for the RUB press release.