Dortmund, 24th April 2020
The heart grows not only through sport, but also through illness. Researchers from Wuerzburg and Dortmund have now discovered a new possible point of attack for drugs to counteract this.
If the heart is heavily loaded, it reacts and grows. This happens, for example, during high sporting activity, especially endurance sports: the heart becomes stronger. In addition to this positive side of heart growth, however, there is also a dark side: If the heart is permanently stressed by a condition such as high blood pressure, the heart grows too much and heart failure can result.
An interdisciplinary team from the Julius Maximilian University (JMU) Wuerzburg and des Leibniz-Instituts für Analytische Wissenschaften (ISAS), led by ISAS Director Kristina Lorenz, has investigated the role of extracellularly regulated kinases (ERK) in pathological heart growth, identifying for the first time a protein sequence that can inhibit this damaging process. With the development of this sequence, the team has identified a potential target for drugs for use in cardiac insufficiency.
It usually takes several stress factors before the proverbial barrel overflows. This is similar in the smallest living units of the body, the cells. There, many protein molecules normally work together to ensure the survival of the cells. If too many stress factors come together, the protein molecules change their function and their cooperation in the cells is disturbed. The consequence: pathological cell growth, which can lead to heart failure. Because: If the enzymes ERK 1 and ERK 2 come close to each other, a biochemical change takes place, a so-called phosphorylation, which can trigger pathological heart growth.
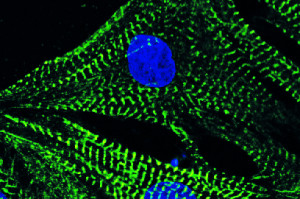
Neonatal cardiomyocytes stained with phalloidin: The figure shows heart muscle cells, blue is the nucleus and green the cytoskeleton. Picture: Constanze Schanbacher and Theresa Brand Neonatal cardiomyocytes stained with phalloidin: The figure shows heart muscle cells, blue is the nucleus and green the cytoskeleton.
© Constanze Schanbacher und Theresa Brand
Healthy cooperation is guaranteed
The team was able to show that maintaining the distance between the ERK particles leads to the prevention of this phosphorylation of the enzymes ERK 1 and ERK 2. This is the key to protecting against diseased cell growth. The teams identified a small protein sequence that keeps these ERK particles at a distance, thus guaranteeing the healthy cooperation of the protein molecules. The researchers named this protein sequence EDI, an acronym for ERK dimerisation inhibitor.
In the future, the principle of EDI could not only help patients suffering from cardiac insufficiency, but could also be applied in oncology. A class of therapeutics for the treatment of tumour diseases also targets the ERK enzymes, but switches off all ERK functions.
Help for cancer patients
Since ERK always has a protective function in the regulation of cellular processes, a complete switch-off of ERK can lead to severe side effects, particularly in the heart. The studies in animal models suggest that the use of EDI preserves the protective ERK effects and only switches off the pathological ERK components. In addition, the team has already been able to show in cell culture that EDI also slows down the growth of cancer cells.
"Now we want to pursue this therapeutic strategy in order to help patients suffering from cardiac insufficiency and the side effects of chemotherapy," said junior researchers Constanze Schanbacher and Theresa Brand giving an outlook on further research.
The work was financially supported by the German Federal Ministry of Education and Research, the Ministry of Innovation, Science and Research of North Rhine-Westphalia and the German Research Foundation.