Dortmund, 16th August 2024
Decoding the unique signature of lipids (fats) is Prof Dr-Ing. Sven Heiles's area of expertise. As Head of the Lipidomics junior research group at ISAS, he is doing all he can to perform in-depth analyses on these biomolecules in tissue or blood, for example. He uses a special mass spectrometry imaging procedure to do this work. Together with his team and researchers from the Justus Liebig University Giessen, Heiles has succeeded in decoding the lipid signature of single cells for the first time. In this interview, the analytical chemist explains the benefits of single-cell analysis and why the researchers chose microglial cells, of all things, as their model.

Sven Heiles holds a professorship at the University of Duisburg-Essen and heads the Lipidomics junior research group at ISAS.
© ISAS
What information can be gleaned from the lipid signatures of single cells?
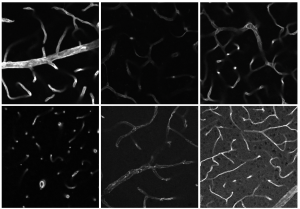
Heiles: The specific pattern created by the quantity, type and spatial distribution of lipids is what we call the lipid signature. It gives us valuable information about the lipid metabolism, which can vary between people who are healthy and those who are ill. Until now we have only been able to decode lipid signatures for larger areas, such as tissue or blood samples. But recently we managed to decode single cells for the first time too. Through the example of microglial cells, we were able to show in a cell culture that genetically identical cells sometimes respond to stimulation completely differently at the lipid level. We saw, for instance, that microglial cells which are no longer functioning correctly accumulate more so called lipid droplets than normal microglial cells. We were able to make these droplet-shaped fat deposits and their molecular composition visible using mass spectrometry imaging, AP MALDI-MSI.
Why did you study microglial cells, of all things?
Heiles: Although microglial cells are incredibly fascinating, for us it was less about the cells themselves and more about their heterogeneous properties. We already know that genetically identical microglial cells are often very different at the transcription and protein level – meaning they have different phenotypes, or observable characteristics. Based on previous research, we expected that these different subtypes would also exhibit differences in terms of lipid metabolism. But until now the technology to prove this just did not exist. We wanted to study the microglial cells to ascertain whether it is possible in principle to make these differences visible using AP MALDI-MSI. Of course, we were also interested in finding out whether our method is sensitive enough and able to image metabolic products that are sufficiently relevant. The hard work was all worthwhile. We delivered the proof of concept and were therefore able to prove that our approach is suitable for investigating subtype heterogeneity amongst cells in a cell culture.
What is the benefit of using AP MALDI-MSI over other imaging procedures?
Heiles: There are a few different procedures for imaging cells in detail. In fluorescence microscopy, for example, specific dyes and the corresponding microscopes are used to make selected structures visible. This is a targeted approach, which allows certain parts of a cell to be visualised selectively. But what happens if we don't even know what we want to see or if no suitable fluorescent dyes exist? That's where AP MALDI-MSI comes in. We don't need any dyes and in principle anything that we are able to ionise, we can make visible. Plus, unlike in fluorescence microscopy, we are not making the dye visible, but the biomolecules themselves.
You've shown that your method works. What's next?
Heiles: Information about the metabolism of single cells could be really exciting, as it could give us a greater understanding of highly heterogeneous conditions such as heart attacks or tumours. Not every cell inside a tumour is identical. Factors such as the local environment and, consequently, the supply of nutrients may vary. Now that we know our method works in principle, we are already working on studying cardiac and tumour cells too. In the long term, however, we want to move away from cell cultures and optimise our method such that we can perform single-cell analyses on complex samples like tissue sections. If we can better understand how nutrients are supplied to single cells under real conditions, that could help to elucidate disease mechanisms related to the metabolism of cells. Ultimately, our technology aims to improve our fundamental understanding of disease models and so to further the development of new treatments, for example, together with partners from clinical practice.
Article Recommendation
Müller, M.A., Zweig, N., Spengler, B., Weinert, M., Heiles, S.
(2023) Lipid Signatures and Inter-Cellular Heterogeneity of Naive and Lipopolysaccharide-Stimulated Human Microglia-like Cells. Analytical Chemistry, 95, 11672 – 11679. https://doi.org/10.1021/acs.analchem.3c01533.
(Interview conducted by Cheyenne Peters and Luisa Becher.)
Find more information on the scientists approach on ISAS Kompakt at "Fatty" signatures: How lipid patterns can be used as a diagnostic tool.