Dortmund, 21st September 2021
Enzymes control the majority of biochemical reactions in the human body. But although they are essential for humans, they can also be the cause of many diseases.
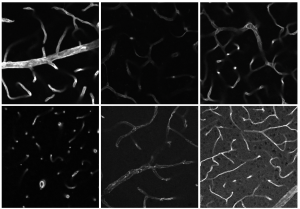
Scientists in the ‘Molecular tools for the investigation of intramembrane proteases’ project at ISAS are studying a class of protein- cleaving enzymes, the proteases. Intramembrane proteases (proteases in the cell membrane), including rhomboid proteases, are the particular focus of the work. The researchers are using synthetic chemistry methods in order to study these proteases. “We’re developing molecular tools, more specifically activity-based and affinity-based probes that can particularly track the enzymes,” reports project leader, Prof Dr Steven Verhelst. This will give the project group some insight into the physiological and potentially pathological role of intramembrane proteases.
Better adaptation of medications to targets in the future
In 2020, Verhelst’s team completed work on ketoamide inhibitors that inhibit rhomboid proteases. They were able to demonstrate that certain parts of the inhibitors, the “primed site” binding elements, are crucial for inhibition. The researchers use these findings together with their chemical toolbox to identify drug binding sites, or targets. Using mass spectrometric analyses, the scientists were also able to demonstrate that the probes they developed, for example based on pepstatin A, an aspartyl protease inhibitor, not only label their protease target but also identify other interaction partners such as substrates. In order to facilitate the quick adaptation of complex small molecules to target structures in the future, they studied the reagents for this “late stage functionalisation” during 2020 and have already started their synthesis.
Exchange via enzymes of the immune defence
Another class of enzymes that the research group has been studying in detail over the past year are the neutrophil serine proteases which influence whether the white blood cells (leukocytes) in the immune system perform a protective or pathological function. In an exchange with the Laboratory for Chemical Biology at the University of Leuven, the group developed probes for these enzymes based on phosphonate and phosphinate esters. They have begun to design a method for fluorescence microscopy in which they hope to visualise the activation process of certain leukocytes with the help of esters.
(Cheyenne Peters)

Prof Dr Steven Verhelst works at ISAS and the Institute of Cell and Molecular Medicine at KU Leuven – University of Leuven, Belgium. He conducts research on chemical biology and chemical proteomics.
© ISAS
What are Targets & Off-Targets?
Every active pharmaceutical ingredient has a target for which it has been developed. These targets can be structures such as enzymes, ion channels or receptor proteins that are involved in the development of a disease and are to be destroyed. Although the molecules are very specific, there are structures (off-targets) to which they bind, even though they are not intended to do so at all. This off-target effect can have an unexpectedly positive impact on a treatment, but often manifests itself as an agonising side effect or concomitant disease for patients.