Dortmund, 8th November 2024
Inflammatory or rejection responses following a heart attack or an organ transplant, for example, are highly complex immunological processes. To understand these in their entirety, it is necessary to analyse the biological structures from the whole organ through individual cells down to the molecular level – just like with a Russian doll. To this end, several ISAS research groups are working on how to combine various microscopy and mass spectrometry techniques within the scope of the project »Al-assisted Imaging of Large Tissues«. Within this project, the interdisciplinary team employs murine samples (from mice) and human samples. These samples are obtained from clinical cooperation partners such as Charité – Universitätsmedizin Berlin and University Hospital Essen. The project’s objective is to perform cross-scale analyses to obtain from the same sample detailed information on the cellular composition and interactions within a tissue.
This makes it possible to perform analyses that are not only more precise but also require fewer resources. Combining various microscopy techniques (including a special clearing method that renders organs transparent (see info box) as well as artificial intelligence (AI) to analyse the images contributes to a significant reduction in the number of samples. In addition, AI experts are working to minimise the amount of energy consumed in data storage and nevertheless increase the analysis quality of the ultra-high resolution microscopic images.
Russian doll principle
The principle applied by the researchers is similar to a Russian doll. Rather than uncovering ever smaller wooden dolls nested inside each other, each step taken by the ISAS team allows them to gain a deeper insight into the biological structures of a sample. Starting with whole organs and going down to the molecular details, this approach facilitates a precise understanding of biological processes.


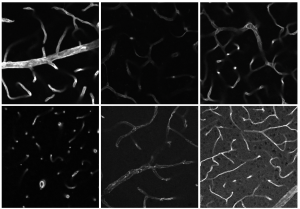
Before going deep into the molecular structures using mass spectrometry, the researchers initially analyse various tissue samples as a whole. Using a lightsheet fluorescence microscope, doctoral candidate Flora Weber examines intact organs such as kidneys or bones from mice, for example. Inside the microscope, a thin sheet of light illuminates the individual layers of a sample rendered transparent by clearing and records an image of each one. The researchers obtain an average of around 500 images per sample, which are then combined into a 3D model on a computer. Afterwards, they reverse the clearing, which means that the same sample can be analysed on a confocal microscope. For this, the researchers do have to slice the organ or tissues, but this is the only way to render the cellular details visible by means of the higher optical resolution of confocal microscopes. Microscopy delivers different data than mass spectrometry, for example. By combining different techniques, the researchers intend, over the long term, to generate what is known as multimodal data. This information consisting of different data types is integrated and analysed at ISAS in cooperation with the junior researcher groups AMBIOM – Analysis of Microscopic BIOMedical Images and Multidimensional Omics Data Analysis.
© ISAS / Hannes Woidich

Dr Martin Stenzel uses the flow cytometer to analyse individual cells at a high throughput. With the help of imaging, he introduces a liquid sample, in this case blood cells from a tumour patient, into the device. Inside the device, a narrow flow of fluid takes it past various laser sources. When the cells pass though the light beam, they scatter the light in characteristic ways. Sensors record the scattered light, including radiation in the visible wavelength range and fluorescence radiation. The signals reveal exactly which cell types and cell components are present in a sample and in what quantities. To integrate flow cytometry into the workflow, the Bioimaging research group is currently developing an alternative way of preparing samples for lightsheet fluorescence microscopy. For this type of microscopy, they have to first fix the samples, i.e. chemically stabilise the cells or tissue structures so that they maintain their shape and position during analysis. The new approach by the researchers aims to achieve a reversible fixing technique so that parts of the same sample can subsequently be analysed using flow cytometry.
© ISAS / Hannes Woidich

The researchers test their workflow using various samples including murine hearts as well as murine and human kidney samples in the context of reperfusion injury. This kind of injury occurs when the blood flow is restored to a tissue following an interruption – for example, following a heart attack or a kidney transplant when closed-off vessels are opened again. The figure shows murine jawbones from a model of medication-related osteonecrosis of the jaw (MRONJ). In this condition, parts of the jawbone die off, due to medication against osteoporosis, for example. Using a clearing technique developed by Prof Dr Anika Grüneboom, the researchers first render the bones transparent for analysis with a lightsheet fluorescence microscope (picture on right). Bone tissue is very heterogeneous: while the cortical bone, the outer layer, is hard, the bone marrow inside is comparatively soft. There are, in addition, cartilage tissue, tendons and muscles on the outer bone interfaces. The various tissue types each require appropriate methods of chemical preparation to make them accessible for the different analytical methods. For this reason, the researchers are investigating an approach that unites the contrasting requirements for sample preparation within the workflow.
© ISAS / Hannes Woidich
Clearing
Tissue and bone can influence light in different ways: by absorbing, reflecting or scattering it. Consequently, researchers must chemically treat a sample before they can examine it in its entirety beyond the surface using a lightsheet fluorescence microscope. For this purpose, Prof Dr Anika Grüneboom has developed a technique that makes the samples transparent using ethyl cinnamate, a naturally occurring aromatic substance. Optical clearing leaves the samples intact and is reversible. This means researchers can subsequently examine the same bone or the same tissue under a confocal microscope, for example.