Dortmund, 29th August 2024
When doctors and scientists at University Hospital Essen (UK Essen) were examining a six-year-old child with the neuromuscular disorder NEDHFBA, they could not imagine the results that one single patient sample would provide. Researchers from ISAS and the Department of Neuropaediatrics at UK Essen worked out how proteins affect the development of this rare condition for the first time. Their extensive analysis could also shed light on other neuromuscular disorders.
The six-year-old boy could smile, but he could not speak. His muscles were so poorly developed that he was not able to sit up unaided. Walking was impossible. When he drifted off to sleep, his eyes stayed half open. The paediatric neurologists in Essen suspected a neurological developmental disorder. An analysis of the boy's genome showed that he had two mutations in a gene called PPP1R21. The protein produced by this gene affects many important processes within cells, including how a number of different proteins interact. Both of the patient's parents were healthy. However, despite being completely unaware of it, both the mother and father had a modified variant of PPP1R21 in their genome as well as a "normal" version. Purely by chance, both had passed down the modified variant to their son. Now present in duplicate, this homozygous mutation exerted its harmful effect on the boy.
Medics group the impairments caused by the PPP1R21 variant together under the abbreviation NEDHFBA, which stands for neurodevelopmental disorder with hypotonia, facial dysmorphism and brain abnormalities. The young patient's dysfunctions were still mild; for example, none of the brain abnormalities typical of NEDHFBA could be observed. Many patients affected by this disorder have a decreased volume of white matter in the brain, an underdeveloped cerebellum and enlarged ventricles (cavities).
Combination of biochemical, microscopic and cytological analyses
"Until now, we knew little about the pathomechanism of NEDHFBA or, in other words, the origin of the disorder," says PD Dr Andreas Roos from the Department of Neuropaediatrics at University Hospital Essen (UK Essen) and the Department of Neurology at the Children's Hospital of Eastern Ontario (Ottawa, Canada). Roos is an Adjunct Professor at the University of Ottawa. He and ISAS researchers working in collaboration with scientists from UK Essen had already proven back in 2021 that an easily accessible cell type, the fibroblast skin cell, offered a good way of studying neuromuscular disorders. The researchers at ISAS now examined the boy's tissue sample to figure out which molecular processes are relevant for NEDHFBA.

PD Dr Andreas Roos is Adjunct Professor at the University of Ottawa and Head of Preclinical Research at the Department of Neuropaediatrics at the University of Essen.
© Privat
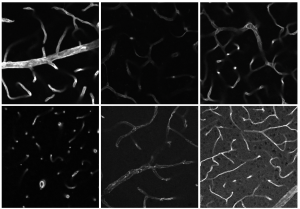
The team used a DNA sequencing method in which specifically the protein-coding regions of the genome – the exons of all the genes – are sequenced, then examined them for mutations. In addition, the researchers created cell cultures of the fibroblasts to track which processes in the cells were not working normally. Using highly sensitive mass spectrometers, the team working with Dr Andreas Hentschel was also able to analyse the boy's proteome, i.e. the complete set of proteins in his cells, qualitatively and quantitatively. "Every analyte in a sample has a particular mass, which can be uniquely assigned. We can then use that to identify the protein in question via mass spectrometry," says Hentschel, summing up the principle of comprehensive proteomic analysis.
The various biochemical, microscopic and cytological investigations showed that mutations in the PPP1R21 gene mean specific proteins are still produced in the patient's cells, but they are present in varying concentrations or are unstable. In total this affects around 18 percent of the proteins that were examined via mass spectrometry, according to the researchers' joint report published in the journal Molecular Neurobiology.
Dysregulation of various proteins
The information obtained about which proteins were affected was revealing as well. On the one hand, it appears the proteasome is overactivated in the young patient's cells. The proteasome is a cellular apparatus that gets rid of unwanted or overaged proteins. According to Roos, this will likely result in an excessive degradation. At the same time, and presumably linked to the overactivated proteasome, the cytoskeleton of the cells – the architecture built from protein fibres – is dysregulated. This in turn affects the organisation of certain structures, also known as cell polarity. "Polarity is tremendously important for nerve cells, or neurons, in particular. It is how the cells know which direction they must grow out to," says Roos.
There are only around a dozen known cases of NEDHFBA worldwide. But the disease falls under the wider umbrella of neuromuscular disorders, which it is estimated affect between seven and 80 children in every 100,0001. This equates to a couple of hundred newborns every year in Germany. "When us researchers study disorders like these, we are basically trying to identify any similarities or recurring patterns," explains Hentschel. He goes on: "Perhaps in various neuromuscular disorders the stability and/or activity of proteins and, consequently, of certain metabolic pathways is repeatedly wrong. If this is the case, we can ask ourselves what needs to happen for these protein functions to work correctly again?"

Dr Andreas Hentschel is a research associate in the ISAS Proteomics research group.
© ISAS
Article Recommendation
Hentschel, A., Meyer, N., Kohlschmidt, N., Groß, C., Sickmann, A., Schara-Schmidt, U., Förster, F., Töpf, A., Christiansen, J., Horvath, R., Vorgerd, M., Thompson, R., Polavarapu, K., Lochmüller, H., Preusse, C., Hannappel, L., Schänzer, A., Grüneboom, A., Gangfuß, A. & Roos, A.
(2023). A Homozygous PPP1R21 Splice Variant Associated with Severe Developmental Delay, Absence of Speech, and Muscle Weakness Leads to Activated Proteasome Function. Molecular Neurobiology, 60:2602–2618.
One basket for multiple disorders
Further studies are vital for these young patients and their families. Usually, the number of patients affected by each individual disorder is too small for the pharmaceutical industry to invest money in developing a treatment for a specific condition. "If we can understand the pathomechanism and it turns out that similar processes have a role to play in several disorders, this could make the market for new drugs large enough to warrant investment," says Roos. In the industry this approach is known as a basket trial, since different disorders are placed into the same basket with a uniform treatment strategy.
In the best-case scenario, however, there will be no need for any new medication if drugs that are already approved for other conditions could also be effective for NEDHFBA. Further research is required in this area.
"A game-changer for our understanding of neuromuscular disorders"
The findings of the fibroblast analysis conducted on the six-year-old NEDHFBA patient garnered a great deal of attention. "The findings are incredibly powerful," says Roos, who as one of the paper's corresponding authors has received enquiries from medics all over the world since it was published. The method employed by Hentschel and his colleagues also enables scientists to predict whether the children of carriers of other diagnosed mutations in the PPP1R21 gene could also be prone to neurological dysfunctions.
"We have laid the foundations and shown at a cellular level how fibroblasts can be used to reveal the pathogenic action of gene mutations in neuromuscular disorders like NEDHFBA," says Roos. "That is not only a game-changer when it comes to our understanding of these disorders, it could also enable us to give specific genetic advice to couples who have known mutations in the PPP1R21 gene and who want to have children."
1 https://www.mdpi.com/journal/children/special_issues/Neuromuscular_Disorders_Children_Adolescents
(Ute Eberle)