Dortmund, 4th November 2021
When Flora Weber is looking at her samples under the microscope, the world around her fades away: A skill that the 25-year-old PhD student has learned while pursuing climbing as a hobby. Since July 1, 2021, she has been conducting research on the medication-induced death of the jaw bone in the Bioimaging working group. Before that, Weber studied biology and completed her master’s degree in the international programme “Integrated Immunology” at the Friedrich-Alexander University Erlangen-Nuremberg. In the interview, the biologist explains why it is so important to concentrate on more than just the details in microscopy.

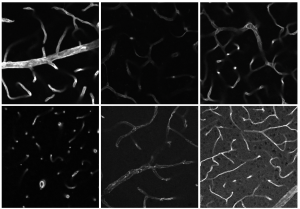
By combining different imaging methods for the analysis of cells, Flora Weber, shown here with the confocal microscope, sees information about the lager contexts and specific details.
© ISAS
Why did you come to ISAS?
Weber: I already got to know Prof Dr Grüneboom, who now leads a working group here at ISAS, during my master’s thesis in Erlangen. There, she introduced me to the imaging methods I use today, for example lightsheet fluorescence microscopy. My thesis was about the bone structure of mice. I mainly dealt with bone cells, namely osteoblasts and osteoclasts. I liked this field of research a lot. That’s why I came to ISAS for my doctorate. Here, I get the chance to continue my research.
What is the aim of your research?
Weber: I study the Medication-Related Osteonecrosis of the Jaw (MRONJ). This is a disease during which parts of the jawbone die – caused, for example, by drugs for the treatment of osteoporosis. The drugs, mostly so-called bisphosphonates, are necessary to prevent bone resorption due to osteoporosis. However, we don’t know yet why they lead to the development of MRONJ. Cancer patients, who have a high risk of osteoporosis because of radiotherapy, also take this drug in high dosages. That’s why they also very often suffer from MRONJ.
In order to understand the mechanism of the disease better, I want to find out how the cells of the bone tissue interact with the cells of the blood supply. I concentrate on the connection of two processes: the forming of new bones (osteogenesis) and the development of blood vessels from pre-existing blood vessels (angiogenesis). I then compare this so-called osteogenic-angiogenic coupling in the jawbone with that in the shinbone, because the shinbone isn’t affected by MRONJ. It isn’t yet known why MRONJ only attacks the jawbone. If we manage to determine differences in the cell structures of the jawbone and other bones, like the shinbone, that would be our chance to prevent the development of MRONJ or to establish therapeutic approaches against osteoporosis that don’t produce the described side-effects.
Which methods of analysis are relevant for your work, what is your approach?
Weber: To better understand the disease in humans, I look at the jaw- and shinbones of mice with MRONJ. I use different microscopes, for example the lightsheet fluorescence microscope or the confocal microscope. Depending on which microscope I use, I employ a clearing protocol devised by Prof Dr Grüneboom, which makes bones transparent. That’s necessary in order to reduce the light scattering during microscopy and to penetrate more deeply into the tissue visually.
Why do you work with different microscopes?
Weber: It’s very important to examine not only the details, but also the larger context. For example, the lightsheet fluorescence microscope is suitable for depicting a whole organ or a complete bone. It takes a lot of single images of the respective layers, which I then combine into a 3D model. Thus, I get a complete overview and can see how the cells differ from each other, for example. After that, I look at specific details with the confocal microscope. In order to do so, I have to cut the bone into slim slices. That’s why this method works without the clearing. By the way, this process is reversible – I can undo the clearing and look at the same sample under different microscopes. That’s very important because I make a point of using resources in a sustainable way.
How do you evaluate the images after that?
Weber: I use different programmes. My main programme is IMARIS, a software for image analysis. To distinguish the different cells, I colour characteristic proteins with fluorescent antibodies as a marker. With surface markers, I can see the cell’s edges, whereas the focus is more on the inner structures in the case of intracellular markers. Depending on the marker, the signal is identified in different ranges of wavelength and displayed in a different colour on the computer. Using this method, I try to find differences in the spatial-temporal distribution of the cells. Ideally, I can see what the structures look like and whether the cells communicate with each other.
What fascinates you about Imaging?
Weber: Imaging delights me, because it’s virtually tangible. An image shows me all the important information at a glance. The spatial distribution of cells or their protein profiles are very hard to depict in a graph. Moreover, I find bones fascinating, because they are very complex. At the beginning, I always thought the immune system only consisted of B cells and T cells - namely those cells that induce an immune response. However, osteoclasts and other cells associated with bones are also part of the immune system. Like other immune cells, they are developed in the bone marrow and react to inflammations and disturbances.
How do you like to spend your time outside the institute?
Weber: I like to meet friends and I do lots of sports. My favourite activity is to go bouldering – if the weather allows it, I love to climb outside. I need to overcome my fears and be ready to test my limits when I go climbing – just like in my research. When doing imaging work in the lab, I also need to tackle challenges and be willing to venture into something new.
(Cheyenne Peters)