Dortmund, 4th August 2022
Toddlers who can barely sit, let alone crawl or walk: Although the disease they suffer from, spinal muscular atrophy (SMA), is rare, patient cases have increased in media coverage during recent years. The reason for the media interest was and is the high cost of a drug approved in Europe in 2020 for SMA: One dose of Zolgensma® (onasemnogene abeparvovec) costs approximately two million euros, making it considerably more expensive than the alternative drug Spinraza® (nusinersen). Zolgensma® is intended to halt progressive muscle weakness after a single dose in patients under two years of age. To assess whether SMA therapies are working and how they are progressing, biomarkers known as progression markers come into play in clinical practice. Scientists at ISAS working on the project »Gene and protein signatures as GPS for patients with neuromuscular diseases« (coordinated by University Hospital Essen) have identified a protein that could help optimise SMA therapy.
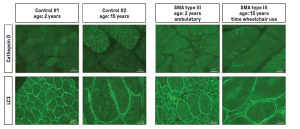
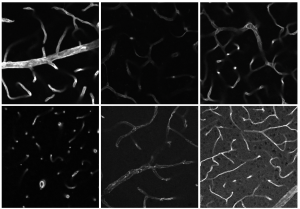
The microscope images show stained muscle biopsies from seven SMA patients aged two and 15 years who had not received any drug therapy. Result: Compared to the control samples from healthy individuals, the protein cathepsin D was downregulated as a uniform pattern in all samples. As a control, the scientists carried out another immunostaining of another protein, LC3. This showed no changes in the muscle cells of SMA patients.
© Department of Pediatric Neurology, Developmental Neurology and Social Pediatrics, Centre for Neuromuscular Disorders in Children and Adolescents, University of Duisburg-Essen
The hereditary disease SMA is a disease of the nerves of the spinal cord and the motor neurons. SMA is triggered by a mutation in the gene for surviving motor neuron 1 (SMN1). This gene is responsible for the production of the »Survival Motor Neuron« (SMN) protein which maintains the health and normal functioning of motor neurons which, in turn, regulate muscle activity by sending signals from the central nervous system – the part of the nervous system that includes the brain and spinal cord. Degeneration of motor neurons leads to a gradual decrease in muscle mass and strength. When neurons are damaged or die, as in SMA, the body releases structural proteins called neurofilaments into the bloodstream or into the cerebospinal fluid (CSF) (brain and spinal cord). “In young SMA patients, the number of neurofilaments is elevated. During treatment with Spinraza® they decrease faster than in untreated patients. However, significant changes are primarily observed in children under one year of age,” says Dr Andreas Hentschel, a member of the Translational Analytics research group.
Cathepsin D as a progression marker
How might a protein help optimise therapy in SMA? “We found that cathepsin D levels in CSF samples decreased in our cohort of SMA patients on Spinraza® therapy,” Hentschel explains.

Dr Andreas Hentschel’s research includes looking for biomarkers with which to monitor therapy and disease progression in SMA patients.
© ISAS
According to the researcher, this decrease appeared in all SMA subtypes and in all age categories of two months or older. In addition, the decrease was more pronounced in patients who exhibited a positive motor response to drug treatment.
“Although our cohort was too small to compare age groups and SMA subtypes within the therapy responder group, we believe these results are very promising. They suggest that the cathepsin D level is also suitable as a biomarker in older SMA patients – in combination with the analysis of the neurofilament light chain protein in adolescents or as a sole marker in adults,” Hentschel summarises. Further validation studies in larger cohorts and with serum samples are needed to assess the applicability of cathepsin D as a progression marker for the various SMA treatments.
(Sara Rebein)
The project entitled »Gene and protein signatures as GPS for patients with neuromuscular diseases« was funded by the State of North Rhine-Westphalia using resources from the 2014-2020 European Regional Development Fund (ERDF), »Investment for Growth and Jobs« (funding reference EFRE-0801301).