Dortmund, 25th March 2022
According to the Federal Statistical Office of Germany, cardiovascular diseases were the cause of more than one third of deaths in the country in 2020. One of these life-threatening cardiovascular diseases is chronic heart failure. If the disease remains untreated, it can progress further and damage the heart as well as other organs such as the lung in the long term. Since January 2020, the project »ChInValue – NRW–China Cooperations: Optimisation of GRK5 Inhibitors for the Therapy of Heart Failure and Heart Hypertrophy« is pursuing a new approach for the drug therapy of chronic heart failure and the abnormal enlargement of the heart’s muscle cells that often goes along with it.
Chronic heart failure can have various causes, for example high blood pressure or a heart attack. In most cases, heart failure is only detected and treated when patients already display symptoms such as a reduced performance, shortness of breath and breast pain. G-protein-coupled receptors (GPCRs) play an important role in the physiological heart function. They are located in the cell membrane and transmit signals to the inside of the cell, for example to regulate the strength and rate of the heartbeat. In cardiovascular diseases, GPCRs are often chronically stimulated. As a result, certain enzymes, the so-called G-protein-coupled receptor kinases (GRK), desensitise the receptors. “In the long run, the overstimulation can lead to a reduced heart function and ultimately to heart failure. That is why we want to develop an agent that specifically inhibits the key enzyme that is upregulated in heart failure, GRK5,” says Miriam Kleindl, PhD student in the working group Cardiovascular Pharmacology at ISAS.

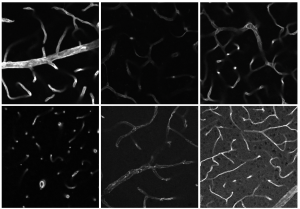
Miriam Kleindl analyses the activation and quantity of proteins of the in vivo GRK study in order to find out how the signalling pathways are regulated.
© ISAS
Preventive and therapeutic approaches show initial successes in the mouse model
In the ChInValue consortium, Kleindl conducts research together with scientists from the Lead Discovery Center GmbH (LDC) in Dortmund and the Chinese project partner Shanghai Jemincare Pharmaceutical Co. At the LDC and at Jemincare, the researchers optimise the inhibitors for the molecular target GRK5 multiparametrically, in medicinal chemistry cycles that consist of organic syntheses as well as tests of the new compounds in biochemical and cellular assays. “In the course of these optimisation cycles, we managed to develop potent, compatible and selective GRK5 inhibitors that meet the qualitative requirements to show activity in therapeutic animal experiments – an important step in the value chain on the path towards a new drug,” Dr Bert Klebl, chief scientific officer and managing director at LDC, reports delightedly.
At ISAS, Kleindl and her colleagues study the agent’s effect in the case of an actual disease in vivo by means of different murine models that replicate heart hypertrophy (increase in the heart’s muscle mass and weight), high blood pressure and heart attacks. “We do not only look at how well the inhibitors block GRK5, but also at whether they influence the progression of the disease,” the human biologist explains. In a first study, Kleindl and her colleagues at ISAS treated some of the animals preventively in order to analyse the inhibitors’ effectiveness – before a heart attack or chronic high blood pressure occurred and caused heart failure. They treated the other animals immediately after the mice fell ill. Afterwards, the scientists studied the heart function in both groups and compared it to a healthy control group. “We discovered that a preventive treatment with one of the GRK5 inhibitors we developed had a positive effect on the animals’ heart function and life span,” Kleindl sums up. The GRK5 inhibitors also show effectiveness in already sick mice.
Consortium strengthens international cooperation
The project partners’ continuous exchange of information enables the researchers at LDC to further develop the inhibitors according to the results of the in vivo studies. Furthermore, data from in vivo compatibility studies (pharmacokinetic studies) feed into the optimisation. These take place at Jemincare, the Chinese project partner. Together, the consortium wants to develop the agent to a level where it can be nominated as a preclinical candidate after the project is finished. In a preclinical phase, the scientists check for possible side effects in order to ensure its safety before the first application in human studies.
About ChInValue
BIO.NRW and BIO Clustermanagement NRW GmbH designed ChInValue in the context of the internationalisation measure ‘NRW-China Cooperations: A Strategic Perspective for Innovative Life Science KMU Value Chains’ (NRW-China Kooperationen: Eine strategische Perspektive für innovative Life-Science-KMU Wertschöpfungsketten). The project lasts three years, until the end of 2022. The Federal Ministry of Education and Research funds the German partners with about one million euros. The funds come from the initiative ‘InterSpiN+: Internationalisation of Leading-Edge Clusters, Forward-Looking Projects and Comparable Networks’ (InterSpiN+: Internationalisierung von Spitzenclustern, Zukunftsprojekten und vergleichbaren Netzwerken).
About the Lead Discovery Center
The Lead Discovery Center GmbH was established in Dortmund in 2008 by the technology transfer organisation Max Planck Innovation GmbH in order to tap into the potential of excellent basic research for the development of new, urgently needed drugs. The Lead Discovery Center takes in promising projects from academic research and typically develops them further up to pharmaceutical leads (proof of concept in model systems). In close cooperation with leading partners from academic research and the industry, the Lead Discovery Center develops an extensive portfolio of projects in the field of small molecule drugs as well as therapeutic antibodies with exceptionally high medicinal and commercial potential.
(Cheyenne Peters)
ChInValue is funded by the Federal Ministry of Education and Research under the funding number 03INT703AB.