Dortmund, 3rd December 2021
Imagine being able to observe the nervous or cardiovascular system from the outside, while it is working. A transparent human being would not only be incredibly exciting, but could certainly also clarify many medical questions about the genesis, early detection or therapy of diseases. After all, biological structures are incredibly complex. They consist of different tissues with various characteristics.
Transparency is Prof Dr Anika Grüneboom’s goal. "We need non-destructive and safe methods that allow us to look deep into the body," explained the immunologist and head of the Bioimaging working group at ISAS. The challenges and opportunities of these methods were the topic of the HealthTech Lecture "The Fluorescence Microscopy Toolbox - Building Bridges to Immunology" of the Leibniz Research Alliance “Leibniz Health Technologies” on November 22, 2021.
No "one size fits all" in microscopy
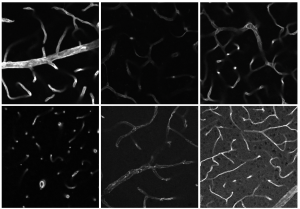
There is a lot to discover in Grüneboom's toolbox. To meet the diverse optical challenges of biological structures, the researcher and her team combine different microscopy techniques. "So far, there is no method that covers all scales in biological samples," said the biologist. For example, to understand an infection and the corresponding immune response, it is necessary to look at the process from different perspectives. One of the imaging techniques Grüneboom uses is fluorescence microscopy (see infobox). This form of light microscopy includes light sheet fluorescence microscopy. Here, a laser illuminates only one thin layer of the sample, for example tissue, at a time, without destroying it. The many individual images are later used to create a 3D model of the entire sample on the computer. However, light sheet fluorescence microscopy alone does not provide a "glass-like" result.
HealthTech-Lecture: Der Werkzeugkasten der Fluoreszenzmikroskopie (Prof. Anika Grüneboom, ISAS)
Grüneboom's patented clearing is used worldwide
Since tissue, for example, can absorb, reflect or scatter light, it prevents a deeper insight beyond the surface without chemical treatment. That is why Grüneboom works with a clearing method she has developed. With the help of cinnamic acid ethyl ester, a naturally occurring aromatic substance, she makes her samples transparent before she analyses them under the microscope. Thus, in 2019, the biologist succeeded in discovering a previously unknown anatomical structure: Blood vessels that run through the cortical bone of mice and serve as small "highways" for immune cells of the bone marrow. The unique feature of Grüneboom's technique, which is being used by researchers all over the world: The clearing can be reversed so that samples are not destroyed. In addition, her method is safe for everyday laboratory use, as it works without any toxic, carcinogenic or explosive chemicals.
Transparent human – no science fiction?
With the right "tools" – in this case, Grüneboom’s clearing – light sheet fluorescence microscopy offers enormous opportunities as a method of analysis. In order to better understand immune diseases or thromboses and to be able to diagnose them more quickly in the future, the Bioimaging group is conducting research on samples from mice. In response to a participant's question about human samples, Grüneboom replied: "In principle, my clearing can also be applied to human tissue samples.”
Artificial intelligence revolutionises the analysis
Grüneboom's method is not only relevant for application-oriented basic research. Fluorescence microscopy can also be used to optimise diagnostic procedures. For example, to detect changes in small vascular clusters (glomeruli) in the kidney. Instead of making histological sections of the kidney and measuring them manually, as is usually the case, an algorithm can recognise, count and analyse each of the approximately 15,000 glomeruli in a kidney from the fluorescence microscope images. "Artificial intelligence allows us to get much more precise statistics because we can not only see a section, but the whole organ," Grüneboom said. She is facing the challenge of analysing the enormously large amounts of data that arise with the new ISAS working group AMBIOM (Analysis of Microscopic BIOMedical Images), headed by Dr. Jianxu Chen, which specialises in artificial intelligence.

© ISAS
In principle, my clearing can also be applied to human tissue samples.
Leibnitz-Institut für Analytische Wissenschaften – ISAS – e. V.
How does fluorescence work?
Fluorescence describes the property of substances to absorb short-wave light and to emit it at a different wavelength. In the process, the electrons of the molecule are temporarily excited before they fall back to their original energy level. This releases energy that we perceive as light. In order to be able to recognise certain structures with a fluorescence microscope, researchers stain samples with fluorescent dyes that have the above-mentioned properties. Some substances have a natural fluorescence, they are autofluorescent.
(Cheyenne Peters)
About Anika Grüneboom
Prof Dr Anika Grüneboom (35) has been heading the Bioimaging research group at ISAS since 2020 and holds a professorship for 'Experimental Biomedical Imaging' at the University of Duisburg-Essen (UDE). Born in Essen, she completed her biology studies at RWTH Aachen University from 2006 to 2011. For her doctorate (2017), she changed to the University Hospital Essen. Before being appointed professor at the UDE, she had been a group leader at the University Hospital Erlangen since 2017, where she investigated rheumatological and immunological issues using imaging techniques. Grüneboom has received several awards for her research.